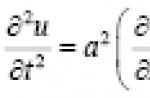Mobile electronics every year, if not a month, is becoming more accessible and more common. Here you have laptops, and PDAs, and digital cameras, and mobile phones, and a lot of all sorts of useful and not very devices. And all these devices are constantly getting new features, more powerful processors, larger color screens, wireless connectivity, while at the same time shrinking in size. But, unlike semiconductor technologies, the power technologies of this mobile menagerie are not at all leaps and bounds.
Conventional accumulators and batteries are clearly not enough to power the latest advances in the electronics industry for any significant amount of time. And without reliable and capacious batteries, the whole point of mobility and wirelessness is lost. So the computer industry is working more and more actively on the problem alternative power sources. And the most promising, to date, direction here are fuel cells.
The basic principle of fuel cells was discovered by the British scientist Sir William Grove in 1839. He is known as the father of the "fuel cell". William Grove generated electricity by changing to extract hydrogen and oxygen. Having disconnected the battery from the electrolytic cell, Grove was surprised to find that the electrodes began to absorb the released gas and generate current. Opening a process electrochemical "cold" combustion of hydrogen was a significant event in the energy sector, and in the future such well-known electrochemists as Ostwald and Nernst played a great role in the development of the theoretical foundations and practical implementation of fuel cells and predicted a great future for them.
Myself the term "fuel cell" (Fuel Cell) appeared later - it was proposed in 1889 by Ludwig Mond and Charles Langer, who were trying to create a device for generating electricity from air and coal gas.
During normal combustion in oxygen, oxidation occurs organic fuel, and the chemical energy of the fuel is inefficiently converted into thermal energy. But it turned out to be possible to carry out an oxidation reaction, for example, hydrogen with oxygen, in an electrolyte environment and, in the presence of electrodes, obtain an electric current. For example, by supplying hydrogen to an electrode in an alkaline environment, we obtain electrons:
2H2 + 4OH- → 4H2O + 4e-
which, passing through the external circuit, enter the opposite electrode, to which oxygen enters and where the reaction takes place: 4e- + O2 + 2H2O → 4OH-
It can be seen that the resulting reaction 2H2 + O2 → H2O is the same as in conventional combustion, but in a fuel cell, or otherwise - in electrochemical generator, an electric current is obtained with great efficiency and partly heat. It should be noted that coal, carbon monoxide, alcohols, hydrazine, and other organic substances can also be used as fuel in fuel cells, and air, hydrogen peroxide, chlorine, bromine, nitric acid, etc. can be used as oxidizing agents.
The development of fuel cells continued vigorously both abroad and in Russia, and then in the USSR. Among the scientists who have made a great contribution to the study of fuel cells, we note V. Jaco, P. Yablochkov, F. Bacon, E. Bauer, E. Justi, K. Kordes. In the middle of the last century, a new assault on fuel cell problems began. This is partly due to the emergence of new ideas, materials and technologies as a result of defense research.
One of the scientists who made a major step in the development of fuel cells was P. M. Spiridonov. Hydrogen-oxygen elements of Spiridonov gave a current density of 30 mA/cm2, which for that time was considered a great achievement. In the 1940s, O. Davtyan created an installation for the electrochemical combustion of generator gas obtained by coal gasification. From each cubic meter of the volume of the element, Davtyan received 5 kW of power.
It was first solid electrolyte fuel cell. It had a high efficiency, but over time, the electrolyte became unusable, and it had to be changed. Subsequently, Davtyan in the late fifties created powerful installation that drives the tractor. In the same years, the English engineer T. Bacon designed and built a fuel cell battery with a total power of 6 kW and an efficiency of 80%, operating on pure hydrogen and oxygen, but the power-to-weight ratio of the battery turned out to be too small - such cells were unsuitable for practical use and too expensive.
In subsequent years, the time of singles passed. The creators of spacecraft became interested in fuel cells. Since the mid-1960s, millions of dollars have been invested in fuel cell research. The work of thousands of scientists and engineers has made it possible to reach new level, and in 1965. The fuel cells were tested in the United States on the Gemini 5 spacecraft, and later on on the Apollo spacecraft for flights to the Moon and under the Shuttle program.
In the USSR, fuel cells were developed at NPO Kvant, also for use in space. In those years, new materials have already appeared - solid polymer electrolytes based on ion-exchange membranes, new types of catalysts, electrodes. And yet, the working current density was small - within 100-200 mA/cm2, and the platinum content on the electrodes was several g/cm2. There were many problems related to durability, stability, safety.
The next stage in the rapid development of fuel cells began in the 1990s. last century and continues to this day. It is caused by the need for new efficient energy sources due, on the one hand, to the global environmental problem of increasing greenhouse gas emissions from the combustion of fossil fuels and, on the other hand, to the depletion of such fuels. Since the end product of hydrogen combustion in a fuel cell is water, they are considered the cleanest in terms of environmental impact. The main problem is only to find an efficient and inexpensive way to produce hydrogen.
Billion-dollar financial investments in the development of fuel cells and hydrogen generators should lead to a technological breakthrough and make their use in everyday life a reality: in cells for cell phones, in cars, in power plants. Already at present such automobile giants as "Ballard", "Honda", "Daimler Chrysler", "General Motors" demonstrate passenger cars and buses running on fuel cells with a power of 50 kW. A number of companies have developed demonstration power plants on fuel cells with solid oxide electrolyte with a power of up to 500 kW. But, despite a significant breakthrough in improving the performance of fuel cells, there are still many problems to be solved related to their cost, reliability, and safety.
In a fuel cell, unlike batteries and accumulators, both the fuel and the oxidizer are fed into it from the outside. The fuel cell is only an intermediary in the reaction and, under ideal conditions, could last almost forever. The beauty of this technology is that, in fact, fuel is burned in the element and the energy released is directly converted into electricity. During direct combustion of fuel, it is oxidized by oxygen, and the heat released in this case is used to perform useful work.
In a fuel cell, as in batteries, the reactions of fuel oxidation and oxygen reduction are spatially separated, and the "burning" process occurs only if the cell supplies current to the load. It's like that diesel power generator, only without diesel and generator. And also without smoke, noise, overheating and with a much higher efficiency. The latter is explained by the fact that, firstly, there are no intermediate mechanical devices and, secondly, the fuel cell is not a heat engine and, as a result, does not obey Carnot's law (that is, its efficiency is not determined by the temperature difference).
Oxygen is used as an oxidizing agent in fuel cells. Moreover, since there is enough oxygen in the air, there is no need to worry about the supply of an oxidizing agent. As for the fuel, it is hydrogen. So, in the fuel cell, the reaction proceeds:
2H2 + O2 → 2H2O + electricity + heat.
The result is useful energy and water vapor. The simplest in its device is proton exchange membrane fuel cell(see figure 1). It works as follows: the hydrogen entering the cell decomposes under the action of a catalyst into electrons and positively charged hydrogen ions H+. Then a special membrane comes into action, which here plays the role of an electrolyte in a conventional battery. By virtue of its chemical composition it allows protons to pass through but retains electrons. Thus, the electrons accumulated on the anode create an excess negative charge, and hydrogen ions create a positive charge on the cathode (the voltage on the element is about 1V).
To create high power, a fuel cell is assembled from many cells. If you turn on the element in the load, then the electrons will flow through it to the cathode, creating a current and completing the process of hydrogen oxidation with oxygen. As a catalyst in such fuel cells, as a rule, platinum microparticles deposited on carbon fiber are used. Due to its structure, such a catalyst passes gas and electricity well. The membrane is usually made from the sulfur-containing polymer Nafion. The thickness of the membrane is tenths of a millimeter. During the reaction, of course, heat is also released, but there is not so much of it, so the operating temperature is maintained in the region of 40-80 ° C.

Fig.1. The principle of operation of the fuel cell
There are other types of fuel cells, mainly differing in the type of electrolyte used. Almost all of them require hydrogen as fuel, so the logical question arises: where to get it. Of course, it would be possible to use compressed hydrogen from cylinders, but immediately there are problems associated with the transportation and storage of this highly flammable gas under high pressure. Of course, it is possible to use hydrogen in bound form as in metal hydride batteries. But still, the task of its extraction and transportation remains, because the infrastructure for hydrogen filling stations does not exist.
However, there is also a solution here - liquid hydrocarbon fuel can be used as a source of hydrogen. For example, ethyl or methyl alcohol. True, a special additional device is already required here - a fuel converter, which at high temperature (for methanol it will be somewhere around 240 ° C) converts alcohols into a mixture of gaseous H2 and CO2. But in this case it is already more difficult to think about portability - such devices are good to use as stationary or, but for compact mobile equipment you need something less bulky.
And here we come to the very device, which is being developed with terrible force by almost all the largest electronics manufacturers - methanol fuel cell(Figure 2).

Fig.2. The principle of operation of the fuel cell on methanol
The fundamental difference between hydrogen and methanol fuel cells is the catalyst used. The catalyst in the methanol fuel cell allows protons to be abstracted directly from the alcohol molecule. Thus, the fuel issue is resolved - methyl alcohol is mass-produced for the chemical industry, it is easy to store and transport, and to charge a methanol fuel cell, it is enough to simply replace the fuel cartridge. True, there is one significant minus - methanol is toxic. In addition, the efficiency of a methanol fuel cell is much lower than that of a hydrogen fuel cell.

Rice. 3. Methanol fuel cell
The most tempting option is to use ethyl alcohol as a fuel, since the production and distribution of alcoholic beverages of any composition and strength is well established throughout the globe. However, the efficiency of ethanol fuel cells is, unfortunately, even lower than that of methanol fuel cells.
As noted over many years of development in the field of fuel cells, built Various types fuel cells. fuel cells classified by electrolyte and type of fuel.
1. Solid polymer hydrogen-oxygen electrolyte.
2. Solid polymer methanol fuel cells.
3. Elements on alkaline electrolyte.
4. Phosphoric acid fuel cells.
5. Fuel cells on molten carbonates.
6. Solid oxide fuel cells.
Ideally, the efficiency of fuel cells is very high, but in real conditions there are losses associated with non-equilibrium processes, such as: ohmic losses due to the specific conductivity of the electrolyte and electrodes, activation and concentration polarization, diffusion losses. As a result, part of the energy generated in fuel cells is converted into heat. The efforts of specialists are aimed at reducing these losses.
The main source of ohmic losses, as well as the reason for the high price of fuel cells, are perfluorinated sulfocationic ion-exchange membranes. Now there are searches for alternative, cheaper proton-conducting polymers. Since the conductivity of these membranes (solid electrolytes) reaches an acceptable value (10 Ω/cm) only in the presence of water, the gases supplied to the fuel cell must be additionally moistened in special device, which also increases the cost of the system. In catalytic gas diffusion electrodes, platinum and some other noble metals are mainly used, and so far no replacement has been found for them. Although the content of platinum in fuel cells is a few mg/cm2, for large batteries, its amount reaches tens of grams.
When designing fuel cells, much attention is paid to the heat removal system, since at high current densities (up to 1 A/cm2) the system self-heats. For cooling, water circulating in the fuel cell through special channels is used, and at low power, air is blown.
So, the modern system of an electrochemical generator, in addition to the fuel cell battery itself, is “overgrown” with many auxiliary devices, such as: pumps, a compressor for supplying air, inlet hydrogen, a gas humidifier, a cooling unit, a gas leakage control system, a converter direct current into a variable, control processor, etc. All this leads to the fact that the cost of the fuel cell system in 2004-2005 was 2-3 thousand $/kW. According to experts, fuel cells will become available for use in transport and in stationary power plants at a price of $50-100/kW.
To introduce fuel cells into everyday life, along with cheaper components, we must expect new original ideas and approaches. In particular, great hopes are associated with the use of nanomaterials and nanotechnologies. For example, several companies recently announced the creation of ultra-efficient catalysts, in particular for the oxygen electrode, based on clusters of nanoparticles from various metals. In addition, there have been reports of non-membrane fuel cell designs in which a liquid fuel (eg, methanol) is fed into the fuel cell along with an oxidizer. Also of interest is the developed concept of biofuel cells operating in polluted waters and consuming dissolved air oxygen as an oxidizer, and organic impurities as fuel.
Experts predict that fuel cells will enter the mass market in the coming years. And indeed, the developers win one after another technical problems, report on successes and present fuel cell prototypes. For example, Toshiba demonstrated a finished methanol fuel cell prototype. It has a size of 22x56x4.5mm and gives a power of about 100mW. One refill of 2 cubes of concentrated (99.5%) methanol is enough for 20 hours of MP3 player operation. Toshiba has released a commercial fuel cell to power mobile phones. Again, the same Toshiba demonstrated a 275x75x40mm laptop power supply element, which allows the computer to work for 5 hours from one charge.
Not far behind Toshiba and another Japanese company - Fujitsu. In 2004, she also introduced an element that works on a 30% aqueous methanol solution. This fuel cell ran on a single 300 ml refill for 10 hours and at the same time produced 15 watts of power.
Casio is developing a fuel cell in which methanol is first processed into a mixture of H2 and CO2 gases in a miniature fuel converter and then fed into the fuel cell. During the demo, the Casio prototype powered a laptop for 20 hours.
Samsung also made a name for itself in the field of fuel cells - in 2004, it demonstrated its 12 W prototype designed to power a laptop. In general, Samsung intends to use fuel cells, first of all, in fourth-generation smartphones.
I must say that Japanese companies generally approached the development of fuel cells very thoroughly. Back in 2003, companies such as Canon, Casio, Fujitsu, Hitachi, Sanyo, Sharp, Sony and Toshiba joined forces to develop a common fuel cell standard for laptops, mobile phones, PDAs and others. electronic devices. American companies, of which there are also many in this market, mostly work under contracts with the military and develop fuel cells to electrify American soldiers.
The Germans are not far behind - the Smart Fuel Cell company sells fuel cells to power a mobile office. The device is called Smart Fuel Cell C25, has dimensions of 150x112x65mm and can produce up to 140 watt-hours on a single charge. This is enough to power the laptop for about 7 hours. Then the cartridge can be replaced and you can continue to work. The size of the methanol cartridge is 99x63x27 mm and it weighs 150g. The system itself weighs 1.1 kg, so you can’t call it completely portable, but still it is a completely finished and convenient device. The company is also developing fuel module to power professional camcorders.
In general, fuel cells have almost entered the mobile electronics market. Manufacturers have to solve the last technical problems before starting mass production.
First, it is necessary to resolve the issue of miniaturization of fuel cells. After all, the smaller the fuel cell, the less power it can produce - so new catalysts and electrodes are constantly being developed that allow, with small sizes, to maximize the working surface. It just comes in handy here latest developments in the field of nanotechnology and nanomaterials (for example, nanotubes). Again, for the miniaturization of the piping of elements (fuel and water pumps, cooling systems and fuel conversion), the achievements of microelectromechanics are increasingly being used.
The second important issue that needs to be addressed is the price. After all, very expensive platinum is used as a catalyst in most fuel cells. Again, some of the manufacturers are trying to make the most of already well-established silicon technologies.
As for other areas of use of fuel cells, fuel cells have already firmly established themselves there, although they have not yet become mainstream either in the energy sector or in transport. Already, many car manufacturers have presented their fuel cell-powered concept cars. Fuel cell buses are running in several cities around the world. Canadian Ballard Power Systems produces a range of stationary generators with power from 1 to 250 kW. At the same time, kilowatt generators are designed to immediately supply one apartment with electricity, heat and hot water.
Energy experts note that in most developed countries, interest in dispersed energy sources is growing rapidly, relatively low power. The main advantages of these autonomous power plants are moderate capital costs during construction, fast commissioning, relatively simple maintenance and good environmental performance. With an autonomous power supply system, investments in power lines and substations are not required. The location of autonomous energy sources directly at the points of consumption not only eliminates losses in the networks, but also increases the reliability of power supply.
Well-known are autonomous energy sources such as small gas turbines ( gas turbine plants), internal combustion engines, wind turbines and solar panels on semiconductors.
Unlike internal combustion engines or coal/gas turbines, fuel cells do not burn fuel. They convert the chemical energy of the fuel into electricity through a chemical reaction. Therefore, fuel cells do not produce large amounts of greenhouse gases released during fuel combustion, such as carbon dioxide (CO2), methane (CH4) and nitrogen oxide (NOx). Fuel cell emissions are water in the form of steam and low levels of carbon dioxide (or no CO2 emissions at all) when hydrogen is used as fuel for the cells. In addition, fuel cells operate silently because they do not include noisy high-pressure rotors and there are no exhaust noises or vibrations during operation.
The fuel cell converts the chemical energy of the fuel into electricity by a chemical reaction with oxygen or another oxidizing agent. Fuel cells consist of an anode ( negative side), cathode ( positive side) and an electrolyte that allows the movement of charges between the two sides of the fuel cell (Figure: circuit diagram fuel cells).
The electrons move from the anode to the cathode through the external circuit, creating DC electricity. Due to the fact that the main difference between different types of fuel cells is the electrolyte, fuel cells are divided according to the type of electrolyte used, i.e. high-temperature and low-temperature fuel cells (TEPM, PMTE). Hydrogen is the most common fuel, but sometimes hydrocarbons such as natural gas and alcohols (i.e. methanol) can also be used. Fuel cells differ from batteries in that they require a constant source of fuel and oxygen/air to keep the chemical reaction going, and they produce electricity as long as they are supplied.
Fuel cells have the following benefits compared to conventional energy sources such as internal combustion engines or batteries:
- Fuel cells are more efficient than diesel or gas engines.
- Most fuel cells are silent when compared to internal combustion engines. Therefore, they are suitable for buildings with special requirements, such as hospitals.
- Fuel cells do not lead to the pollution caused by burning fossil fuels; for example, the only by-product of hydrogen fuel cells is water.
- If hydrogen is obtained from the electrolysis of water provided by a renewable energy source, then when using fuel cells, no greenhouse gas is released throughout the entire cycle.
- Fuel cells do not require conventional fuels such as oil or gas, so economic dependence on oil-producing countries can be removed and greater energy security achieved.
- Fuel cells are not dependent on power grids, as hydrogen can be produced anywhere that water and electricity are available, and the produced fuel can be distributed.
- When using stationary fuel cells to produce energy at the point of consumption, decentralized energy networks can be used, which are potentially more stable.
- Low temperature fuel cells (TEPM, PMTE) have low level heat transfer, making them ideal for a variety of applications.
- Higher temperature fuel cells produce high quality process heat together with electricity and are well suited for cogeneration (such as co-production heat and electricity for residential buildings).
- The run time is much longer than the run time of batteries, since only more fuel is needed to increase the run time, and no increase in plant productivity is required.
- Unlike batteries, fuel cells have a "memory effect" when they are refueled.
- Maintenance of fuel cells is simple as they do not have large moving parts.
The most common fuel for fuel cells is hydrogen, as it does not emit harmful pollutants. However, other fuels can be used, and natural gas fuel cells are considered an efficient alternative when natural gas is available at competitive prices. In fuel cells, the flow of fuel and oxidants passes through electrodes that are separated by an electrolyte. This causes a chemical reaction that produces electricity; there is no need to burn fuel or add thermal energy, which is usually the case with traditional methods of generating electricity. When using natural pure hydrogen as a fuel, and oxygen as an oxidizing agent, as a result of the reaction that occurs in the fuel cell, water, thermal energy and electricity are produced. When used with other fuels, fuel cells emit very low pollutant emissions and produce high quality, reliable electricity.
The advantages of natural gas fuel cells are as follows:
- Benefits for the environment- Fuel cells are a clean method of generating electricity from fossil fuels. Whereas fuel cells running on pure hydrogen and oxygen produce only water, electricity and heat; other types of fuel cells emit negligible amounts of sulfur compounds and very low levels of carbon dioxide. However, the carbon dioxide emitted by fuel cells is concentrated and can easily be captured instead of being released into the atmosphere.
- Efficiency- Fuel cells convert the energy available in fossil fuels into electrical energy much more efficiently than conventional fuel-burning electricity generation methods. This means that to produce the same amount of electricity, it takes less fuel. According to the National Energy Technology Laboratory 58, fuel cells (in combination with natural gas turbines) can be produced that will operate in the power range from 1 to 20 MWe with an efficiency of 70%. This efficiency is much higher than the efficiency that can be achieved with traditional methods of power generation in the specified power range.
- Production with distribution- Fuel cells can be produced in very small sizes; this allows them to be placed in places where electricity is required. This applies to residential, commercial, industrial and even vehicle installations.
- Reliability- Fuel cells are completely enclosed devices with no moving parts or complex machinery. This makes them reliable sources of electricity, capable of operating for many hours. In addition, they are almost silent and safe sources of electricity. Also in fuel cells there are no surges of electricity; this means that they can be used in cases where a constantly working, reliable source of electricity is needed.
Until recently, less popular were fuel cells (FC), which are electrochemical generators, capable of converting chemical energy into electrical energy, bypassing the processes of combustion, the conversion of thermal energy into mechanical energy, and the latter into electrical energy. Electrical energy is generated in fuel cells due to the chemical reaction between the reducing agent and the oxidizing agent, which are continuously supplied to the electrodes. The reducing agent is most often hydrogen, the oxidizing agent is oxygen or air. The combination of a fuel cell stack and devices for supplying reagents, removing reaction products and heat (which can be utilized) is an electrochemical generator.
In the last decade of the 20th century, when power supply reliability and environmental concerns were of particular importance, many firms in Europe, Japan, and the United States began to develop and manufacture several variants of fuel cells.
The simplest are alkaline fuel cells, from which the development of this type of autonomous energy sources began. Working temperature in these fuel cells is 80-95°C, the electrolyte is a 30% solution of caustic potassium. Alkaline fuel cells operate on pure hydrogen.
Last time widespread received a PEM fuel cell with proton exchange membranes (with a polymer electrolyte). The operating temperature in this process is also 80-95°C, but a solid ion-exchange membrane with perfluorosulfonic acid is used as the electrolyte.
Admittedly, the most attractive commercially is the PAFC phosphoric acid fuel cell, which achieves an efficiency of 40% in generating electricity alone, and -85% when using the generated heat. The operating temperature of this fuel cell is 175–200°C, the electrolyte is liquid phosphoric acid impregnating silicon carbide bonded with Teflon.
The cell package is equipped with two porous graphite electrodes and ortho-phosphoric acid as an electrolyte. The electrodes are coated with a platinum catalyst. In the reformer, natural gas, when interacting with steam, passes into hydrogen and CO, which is additionally oxidized to CO2 in the converter. Further, under the influence of the catalyst, hydrogen molecules dissociate into H ions at the anode. The electrons released in this reaction are directed through the load to the cathode. At the cathode, they react with hydrogen ions diffusing through the electrolyte and with oxygen ions, which are formed as a result of the catalytic oxidation of air oxygen at the cathode, eventually forming water.
Fuel cells with molten carbonate of the MCFC type also belong to promising types of fuel cells. This fuel cell, when operating on methane, has an efficiency of 50-57% for electricity. Operating temperature 540-650°C, electrolyte - molten carbonate of potassium and sodium alkali in a shell - a matrix of lithium-aluminum oxide LiA102.
And, finally, the most promising fuel element is SOFC. It is a solid oxide fuel cell that uses any gaseous fuel and is most suitable for relatively large installations. Its energy efficiency is 50-55%, and when used in combined cycle plants, up to 65%. Operating temperature 980-1000°C, electrolyte - solid zirconium, stabilized with yttrium.
On fig. 2 shows a 24-cell SOFC battery developed by Siemens Westinghouse Power Corporation (SWP - Germany). This battery is the basis of an electrochemical generator powered by natural gas. The first demonstration tests of a power plant of this type with a power of 400 W were carried out as early as 1986. In subsequent years, the design of solid oxide fuel cells was improved and their power increased.
The demonstration tests of the plant with a capacity of 100 kW put into operation in 1999 were the most successful. The power plant confirmed the possibility of obtaining electricity with high efficiency (46%), and also showed high stability of characteristics. Thus, the possibility of operating the power plant for at least 40 thousand hours with an acceptable drop in its power was proved.
In 2001, a new power plant based on solid oxide elements was developed, operating at atmospheric pressure. The battery (electrochemical generator) with a power plant capacity of 250 kW with combined generation of electricity and heat included 2304 solid oxide tubular elements. In addition, the plant included an inverter, a regenerator, a fuel (natural gas) heater, a combustion chamber for air heating, a heat exchanger for heating water using the heat of flue gases, and other auxiliary equipment. At the same time, the overall dimensions of the installation were quite moderate: 2.6x3.0x10.8 m.
Some progress in the development of large fuel cells has been achieved by Japanese specialists. Research work was started in Japan as early as 1972, but significant progress was made only in the mid-1990s. Experimental fuel cell modules had a power of 50 to 1000 kW, with 2/3 of them running on natural gas.
In 1994, a 1 MW fuel cell plant was built in Japan. With a total efficiency factor (with steam and hot water generation) equal to 71%, the installation had an efficiency factor for electricity supply of at least 36%. Since 1995, according to press reports, an 11 MW phosphoric acid fuel cell power plant has been operating in Tokyo, and general power produced fuel cells by 2000 reached 40 MW.
All the installations listed above belong to the industrial class. Their developers are constantly striving to increase the power of the units in order to improve the cost characteristics (specific costs per kW of installed capacity and the cost of generated electricity). But there are several companies that set a different goal: to develop the simplest installations for domestic consumption, including individual power supplies. And in this area there are significant achievements:
- Plug Power LLC developed a 7kW fuel cell unit to power a home;
- H Power Corporation produces 50-100 W battery chargers used in transport;
- Intern company. Fuel Cells LLC manufactures 50-300W vehicles and personal power supplies;
- Analytic Power Inc. has developed 150W personal power supplies for the US Army, as well as 3kW to 10kW fuel cell home power supplies.
What are the advantages of fuel cells that encourage numerous companies to invest heavily in their development?
Apart from high reliability electrochemical generators have a high efficiency factor, which distinguishes them favorably from steam turbine plants and even from plants with simple cycle gas turbines. An important advantage of fuel cells is the convenience of their use as dispersed energy sources: the modular design allows you to connect in series any number of individual cells to form a battery - an ideal quality for increasing power.
But the most important argument in favor of fuel cells is their environmental performance. The emissions of NOX and CO from these installations are so small that, for example, the county air quality authorities in the regions (where environmental control regulations are the most stringent in the US) do not even mention this equipment in all requirements regarding the protection of the atmosphere.
Numerous advantages of fuel cells, unfortunately, cannot currently outweigh their only drawback - high cost. In the USA, for example, the specific capital costs for the construction of a power plant, even with the most competitive fuel cells, are approximately $3,500/kW. Although the government is providing a $1,000/kWh subsidy to stimulate demand for this technology, the cost of building such facilities remains quite high. Especially when compared with the capital costs for the construction of a mini-CHP with gas turbines or with internal combustion engines of the megawatt power range, which are approximately $500/kW.
IN last years Some progress has been made in reducing the costs of FC installations. The construction of power plants with fuel cells based on phosphoric acid with a capacity of 0.2-1.0 MW, which was mentioned above, cost 1,700 dollars / kW. The cost of energy production at such installations in Germany, when used for 6000 hours per year, is calculated to be 7.5-10 cents / kWh. The 200 kW PC25 plant operated by Hessische EAG (Darmstadt) also has good economic performance: the cost of electricity, including depreciation, fuel and plant maintenance costs, totaled 15 cents/kWh. The same indicator for TPPs on lignite was 5.6 cents/kWh in the power company, 4.7 cents/kWh on hard coal, 4.7 cents/kWh for combined cycle plants and diesel power plants- 10.3 cents/kWh.
The construction of a larger fuel cell plant (N=1564 kW), operating since 1997 in Cologne, required specific capital costs in the amount of 1500-1750 USD/kW, but the cost of the actual fuel cells was only 400 USD/kW
All of the above shows that fuel cells are perspective view energy-producing equipment for both industry and autonomous installations in the domestic sector. The high efficiency of gas use and excellent environmental performance give reason to believe that after solving the most important task - cost reduction - this type of power equipment will be in demand on the market autonomous systems heat and power supply.
fuel cell- what it is? When and how did he appear? Why is it needed and why are they so often talked about in our time? What are its scope, characteristics and properties? Unstoppable progress requires answers to all these questions!
What is a fuel cell?
fuel cell- this is a chemical current source or an electrochemical generator, this is a device for converting chemical energy into electrical energy. In modern life chemical sources current are used everywhere and are batteries for mobile phones, laptops, PDAs, as well as batteries in cars, uninterruptible power supplies, etc. The next stage in the development of this area will be the widespread distribution of fuel cells, and this is an undeniable fact.
History of fuel cells
The history of fuel cells is another story of how the properties of matter, once discovered on Earth, were widely used far in space, and at the turn of the millennium they returned from heaven to Earth.
It all started in 1839 when the German chemist Christian Schönbein published the principles of the fuel cell in the Philosophical Journal. In the same year, an Englishman, an Oxford graduate, William Robert Grove, designed a galvanic cell, later called the Grove galvanic cell, which is also recognized as the first fuel cell. The very name "fuel cell" was given to the invention in the year of its anniversary - in 1889. Ludwig Mond and Karl Langer are the authors of the term.
A little earlier, in 1874, Jules Verne, in The Mysterious Island, predicted the current energy situation, writing that "Water will one day be used as a fuel, hydrogen and oxygen, of which it is composed, will be used."
Meanwhile, the new technology of power supply was gradually improved, and starting from the 50s of the XX century, not a year passed without the announcement of the latest inventions in this area. In 1958, the first tractor powered by fuel cells appeared in the United States, in 1959. 5KW power supply for welding machine was released, etc. In the 70s, hydrogen technology took off into space: airplanes appeared and rocket engines on hydrogen. In the 1960s, RSC Energia developed fuel cells for the Soviet lunar program. The Buran program also did not do without them: alkaline 10 kW fuel cells were developed. And towards the end of the century, fuel cells crossed zero altitude above sea level - based on them, developed electricity supply German submarine. Returning to Earth, in 2009 the first locomotive was put into operation in the USA. Naturally, on fuel cells.
In all the beautiful history of fuel cells, what is interesting is that the wheel is still the unparalleled invention of mankind in nature. The thing is that fuel cells are similar in their structure and principle of operation to a biological cell, which, in fact, is a miniature hydrogen-oxygen fuel cell. As a result, man once again invented what nature has been using for millions of years.
The principle of operation of fuel cells
The principle of operation of fuel cells is obvious even from the school curriculum in chemistry, and it was he who was laid down in the experiments of William Grove in 1839. The thing is that the process of water electrolysis (water dissociation) is reversible. Just as it is true that when an electric current is passed through water, the latter is split into hydrogen and oxygen, so the reverse is also true: hydrogen and oxygen can be combined to produce water and electricity. In Grove's experiment, two electrodes were placed in a chamber into which limited portions of pure hydrogen and oxygen were supplied under pressure. Due to the small volumes of gas, as well as due to the chemical properties of the carbon electrodes, a slow reaction took place in the chamber with the release of heat, water, and, most importantly, with the formation of a potential difference between the electrodes.
The simplest fuel cell consists of a special membrane used as an electrolyte, on both sides of which powdered electrodes are applied. Hydrogen enters one side (anode) and oxygen (air) enters the other (cathode). Each electrode has a different chemical reaction. At the anode, hydrogen breaks down into a mixture of protons and electrons. In some fuel cells, the electrodes are surrounded by a catalyst, usually made of platinum or other noble metals, to aid in the dissociation reaction:
2H 2 → 4H + + 4e -
where H 2 is a diatomic hydrogen molecule (the form in which hydrogen is present as a gas); H + - ionized hydrogen (proton); e - - electron.
On the cathode side of the fuel cell, protons (passed through the electrolyte) and electrons (which passed through the external load) recombine and react with the oxygen supplied to the cathode to form water:
4H + + 4e - + O 2 → 2H 2 O
Overall reaction in the fuel cell is written as follows:
2H 2 + O 2 → 2H 2 O
The operation of a fuel cell is based on the fact that the electrolyte passes protons through itself (toward the cathode), but electrons do not. The electrons move towards the cathode along the outer conducting circuit. This movement of electrons is the electrical current that can be used to power an external device connected to the fuel cell (a load such as a light bulb):

In their work, fuel cells use hydrogen fuel and oxygen. The easiest way is with oxygen - it is taken from the air. Hydrogen can be supplied directly from a certain container or by separating it from an external source of fuel (natural gas, gasoline or methyl alcohol - methanol). In the case of an external source, it must be chemically converted to extract the hydrogen. Currently, most of the fuel cell technologies being developed for portable devices use methanol.
Fuel Cell Characteristics
they only operate as long as the fuel and oxidizer are supplied from an external source (i.e. they cannot store electrical energy),
the chemical composition of the electrolyte does not change during operation (the fuel cell does not need to be recharged),
they are completely independent of electricity (while conventional batteries store energy from the grid).
Fuel cells are analogous to existing batteries in the sense that in both cases electrical energy is obtained from chemical energy. But there are also fundamental differences:
Each fuel cell creates voltage in 1V. More voltage is achieved by connecting them in series. The increase in power (current) is realized through parallel connection cascades of series-connected fuel cells.
For fuel cells no hard limit on efficiency, as in thermal machines(The efficiency of the Carnot cycle is the maximum possible efficiency among all heat engines with the same minimum and maximum temperatures).
High efficiency achieved through the direct conversion of fuel energy into electricity. If fuel is first burned in diesel generator sets, the resulting steam or gas turns a turbine or internal combustion engine shaft, which in turn turns an electric generator. The result is an efficiency of a maximum of 42%, more often it is about 35-38%. Moreover, due to the many links, as well as due to thermodynamic limitations on the maximum efficiency of heat engines, the existing efficiency is unlikely to be raised higher. For existing fuel cells Efficiency is 60-80%,
Efficiency almost does not depend on the load factor,
The capacity is several times higher than existing batteries
Complete no environmentally harmful emissions. Only clean water vapor and thermal energy are emitted (unlike diesel generators, which have polluting emissions and require them to be removed).
Types of fuel cells
fuel cells classified on the following grounds:
by fuel used
working pressure and temperature,
according to the nature of the application.
In general, there are the following fuel cell types:
Solid-oxide fuel cells (SOFC);
Fuel cell with proton exchange membrane (Proton-exchange membrane fuel cell - PEMFC);
Reversible Fuel Cell (RFC);
Direct methanol fuel cell (Direct-methanol fuel cell - DMFC);
Melt carbonate fuel cell (Molten-carbonate fuel cells - MCFC);
Phosphoric acid fuel cells (PAFC);
Alkaline fuel cells (AFC).
One of the types of fuel cells operating at normal temperatures and pressures using hydrogen and oxygen are elements with an ion exchange membrane. The resulting water does not dissolve the solid electrolyte, flows down and is easily removed.
Fuel Cell Problems
The main problem of fuel cells is related to the need for "packaged" hydrogen, which could be freely purchased. Obviously, the problem should be solved over time, but so far the situation causes a slight smile: what comes first - the chicken or the egg? Fuel cells are not yet advanced enough to build hydrogen plants, but their progress is unthinkable without these plants. Here we also note the problem of the source of hydrogen. Hydrogen is currently produced from natural gas, but rising energy costs will also increase the price of hydrogen. At the same time, the presence of CO and H 2 S (hydrogen sulfide) is inevitable in hydrogen from natural gas, which poison the catalyst.
Common platinum catalysts use a very expensive and irreplaceable metal in nature - platinum. However, this problem is planned to be solved by using catalysts based on enzymes, which are a cheap and easily produced substance.
Heat is also a problem. Efficiency will increase sharply if the generated heat is directed to a useful channel - to produce thermal energy for the heat supply system, to use it as waste heat in absorption refrigerating machines and so on.
Methanol Fuel Cells (DMFC): Real Application
Direct Methanol Fuel Cells (DMFC) are of the highest practical interest today. A Portege M100 laptop running on a DMFC fuel cell looks like this:

A typical DMFC circuit contains, in addition to the anode, cathode and membrane, several additional components: a fuel cartridge, a methanol sensor, a fuel circulation pump, an air pump, a heat exchanger, etc.
The operating time, for example, of a laptop compared to batteries is planned to be increased by 4 times (up to 20 hours), a mobile phone - up to 100 hours in active mode and up to six months in standby mode. Recharging will be done by adding a portion of liquid methanol.
The main task is to find options for using the methanol solution with its highest concentration. The problem is that methanol is a fairly strong poison, lethal in doses of several tens of grams. But the concentration of methanol directly affects the duration of work. If a 3-10% methanol solution was previously used, then mobile phones and PDAs using a 50% solution have already appeared, and in 2008, in laboratory conditions, specialists from MTI MicroFuel Cells and, a little later, Toshiba, obtained fuel cells that work on pure methanol.
Fuel cells are the future!
Finally, the fact that the international organization IEC (International Electrotechnical Commission), which defines industrial standards for electronic devices, has already announced the creation of a working group to develop an international standard for miniature fuel cells, speaks of the obvious great future of fuel cells.
The traditional internal combustion engine (ICE) has a number of significant drawbacks, which forces scientists to look for a worthy replacement for it. The most popular option for such an alternative is the electric motor, but it is not the only one that can compete with internal combustion engines. This article will focus on the hydrogen engine, which is rightfully considered the future of the automotive industry and can solve the problem of harmful emissions and the high cost of fuel.
Short story
Despite the fact that the preservation of the environment has only now become a mass problem, scientists have thought about changing the standard internal combustion engine before. So, a hydrogen-powered motor “saw the world” back in 1806, which was facilitated by the French inventor Francois Isaac de Rivaz (he produced hydrogen by electrolysis of water).
Several decades passed, and in England the first patent for a hydrogen engine was issued (1841), and in 1852 German scientists designed an internal combustion engine that could run on an air-hydrogen mixture.

A little later, during the blockade of Leningrad, when gasoline was a scarce product, and hydrogen was available in fairly large quantities, technician Boris Shelishch suggested using an air-hydrogen mixture for the operation of barrage balloons. After that, all internal combustion engines of balloon winches were transferred to hydrogen power, and the total number of hydrogen-powered machines reached 600 units.
In the first half of the 20th century, public interest in hydrogen engines was not great, but with the advent of the fuel and energy crisis of the 1970s, the situation changed dramatically. In particular, in 1879, BMW released the first car that ran quite successfully on hydrogen (without explosions and water vapor escaping from the exhaust pipe).
Following BMW, other major automakers began to work in this direction, and by the end of the last century, almost every self-respecting auto company already had the concept of developing a hydrogen-powered car. However, with the end of the oil crisis, public interest in alternative fuel sources has also faded, although in our time it is starting to awaken again, fueled by environmentalists fighting to reduce the toxicity of car exhaust gases.
Moreover, energy prices and the desire to gain fuel independence only contribute to theoretical and practical research by scientists from many countries of the world. The most active companies are BMW, General Motors, Honda Motor, Ford Motor.
Interesting fact! Hydrogen is the most common element in the universe, but it will be very difficult to find it in its pure form on our planet.
The principle of operation and types of hydrogen engine
The main difference between a hydrogen plant and traditional engines is a method of supplying fuel liquid and subsequent ignition of the working mixture. At the same time, the principle of transformation of the reciprocating movements of the crank mechanism into useful work remains unchanged. Considering that the combustion of petroleum fuel occurs rather slowly, fuel-air mixture fills the combustion chamber before the piston reaches its extreme top position(so-called top dead center).
 The rapid reaction of hydrogen makes it possible to move the injection time closer to the moment when the piston begins to return to bottom dead center. It should be noted that the pressure in the fuel system will not necessarily be high.
The rapid reaction of hydrogen makes it possible to move the injection time closer to the moment when the piston begins to return to bottom dead center. It should be noted that the pressure in the fuel system will not necessarily be high.
If ideal operating conditions are created for a hydrogen engine, then it can have fuel system nutrition closed type when the mixture formation process will take place without the participation of atmospheric air flows. In this case, after the compression stroke, water vapor remains in the combustion chamber, which, passing through the radiator, condenses and turns back into ordinary water.
However, the use of this type of device is possible only when the vehicle has an electrolyzer that separates hydrogen from water for its re-reaction with oxygen. On this moment it is extremely difficult to achieve such results. For stable operation of engines, it is used, and its evaporation is part of the exhaust gases.
Therefore, the trouble-free launch of the power plant and its stable operation on explosive gas without the use of atmospheric air is still an impossible task. There are two options for automotive hydrogen plants:units operating on the basis of hydrogen fuel cells, and hydrogen internal combustion engines.
Power plants based on hydrogen fuel cells
The principle of operation of fuel cells is based on physical and chemical reactions. In fact, these are the same lead rechargeable batteries, but the efficiency of a fuel cell is slightly higher than that of a battery, and is about 45% (sometimes more).
 A membrane (conducts only protons) is placed in the body of the hydrogen-oxygen fuel cell, separating the chamber with the anode and the chamber with the cathode. Hydrogen enters the anode chamber, and oxygen enters the cathode chamber. Each electrode is pre-coated with a catalyst layer, which is often platinum. When exposed to it, molecular hydrogen begins to lose electrons.
A membrane (conducts only protons) is placed in the body of the hydrogen-oxygen fuel cell, separating the chamber with the anode and the chamber with the cathode. Hydrogen enters the anode chamber, and oxygen enters the cathode chamber. Each electrode is pre-coated with a catalyst layer, which is often platinum. When exposed to it, molecular hydrogen begins to lose electrons.
At the same time, protons pass through the membrane to the cathode and, under the influence of the same catalyst, combine with electrons coming from outside. As a result of the reaction, water is formed, and the electrons from the anode chamber move to the electrical circuit connected to the motor. Simply put, we get an electric current, which feeds the engine.
Fuel cell-based hydrogen engines are currently used on Niva vehicles equipped with the Antel-1 power plant and Lada 111 vehicles with the Antel-2 unit, which were developed by Ural engineers. In the first case, one charge is enough for 200 km, and in the second - for 350 km.
It should be noted that due to the high cost of metals (palladium and platinum) included in the design of such hydrogen engines, such installations are very expensive, which significantly increases the price of the vehicle on which they are installed.
Do you know?Specialists Toyota started working with fuel cell technology 20 years ago. That's when the project started. hybrid car Prius.
Hydrogen internal combustion engines
 This type of power plant is very similar to propane engines common today, so to switch from propane to hydrogen fuel, it is enough to simply reconfigure the engine. There are already many examples of such a transition, but it must be said that in this case the efficiency will be somewhat lower than when using fuel cells. At the same time, less hydrogen energy is required to obtain 1 kW of hydrogen energy, which fully compensates for this disadvantage.
This type of power plant is very similar to propane engines common today, so to switch from propane to hydrogen fuel, it is enough to simply reconfigure the engine. There are already many examples of such a transition, but it must be said that in this case the efficiency will be somewhat lower than when using fuel cells. At the same time, less hydrogen energy is required to obtain 1 kW of hydrogen energy, which fully compensates for this disadvantage.
The use of this substance in a conventional internal combustion engine will cause a number of problems. Firstly, the high compression temperature will "cause" the hydrogen to react with the metal parts of the engine or even the engine oil. Secondly, even a small leak on contact with a hot exhaust manifold will definitely cause a fire.
For this reason, only power units rotary type, as their design reduces the risk of fire due to the distance between the intake and exhaust manifolds. In any case, all problems have so far been overcome, which makes it possible to consider hydrogen as a fairly promising fuel.
A good example of a hydrogen-powered vehicle is the experimental BMW 750hL sedan, a concept that was unveiled back in the early 2000s. The car is equipped with a twelve-cylinder engine that runs on rocket fuel and allows you to accelerate the car to 140 km / h. Hydrogen in liquid form is stored in a special tank, and one supply is enough for 300 kilometers. If it is completely consumed, the system automatically switches to gasoline power.
Hydrogen engine in today's market
Recent research by scientists in the field of operating hydrogen engines has shown that not only are they very environmentally friendly (like electric motors), but they can be very efficient in terms of performance. Moreover, by technical indicators hydrogen power plants outperform their electric counterparts, which has already been proven (for example, Honda Clarity).
 Also It should be noted that, unlike Tesla Powerwall systems, hydrogen analogues have one significant disadvantage:
it will no longer be possible to charge the battery using solar energy, but instead you will have to look for a special gas station, which today, even on a global scale, there are not so many.
Also It should be noted that, unlike Tesla Powerwall systems, hydrogen analogues have one significant disadvantage:
it will no longer be possible to charge the battery using solar energy, but instead you will have to look for a special gas station, which today, even on a global scale, there are not so many.
Now Honda Clarity has been released in a fairly limited batch, and you can buy a car only in the Land of the Rising Sun, since the vehicle will appear in Europe and America only at the end of 2016.
Interesting to know!Power Exporter 9000 generator (may be part of complete set of Honda Clarity) is able to power all home appliances for almost a whole week.
Also in our time, other vehicles using hydrogen fuel are being produced. These include the Mazda RX-8 hydrogen and BMW Hydrogen 7 (hybrids running on liquid hydrogen and gasoline), as well as Ford E-450 and MAN buses. lion city bus.
Among cars the most prominent representatives of hydrogen vehicles today are cars Mercedes-Benz GLC F-Cell(there is the possibility of recharging from a conventional household network, and the total power reserve is about 500 km), Toyota Mirai(works only on hydrogen, and one refueling should be enough for 650 km of travel) and Honda FCX Clarity(the declared power reserve reaches 700 km). But that's not all, because hydrogen-powered vehicles are also produced by other companies, such as Hyundai (Tucson FCEV).
Advantages and disadvantages of hydrogen engines
 With all its advantages, it cannot be said that hydrogen transport is devoid of certain disadvantages. In particular, it must be understood that the combustible form of hydrogen at room temperature and normal pressure is in the form of a gas, which causes certain difficulties in the storage and transportation of such fuel. That is, there is a serious problem of designing safe reservoirs for hydrogen used as a fuel for cars.
With all its advantages, it cannot be said that hydrogen transport is devoid of certain disadvantages. In particular, it must be understood that the combustible form of hydrogen at room temperature and normal pressure is in the form of a gas, which causes certain difficulties in the storage and transportation of such fuel. That is, there is a serious problem of designing safe reservoirs for hydrogen used as a fuel for cars.
In addition, cylinders containing this substance require periodic inspection and certification, which can only be carried out by qualified and licensed personnel. Also, the high cost of servicing a hydrogen engine should be added to these problems, not to mention the very limited number of filling stations (at least in our country).
Don't forget that the hydrogen plant increases the weight of the car, which may make it not as maneuverable as you would like it to be. Therefore, given all of the above, think carefully: is it worth buying a hydrogen vehicle, or is it better to wait with it for now.
However, it must be said that there are many advantages in such a solution. Firstly, your car will not pollute the environment with toxic exhaust gases, Secondly, mass production of hydrogen could help solve the problem of fluctuating fuel prices and disruptions in the supply of conventional fuel liquids.
In addition, pipeline networks for methane have already been built in many countries, and it is not difficult to adapt them for pumping hydrogen with subsequent delivery to gas stations. Hydrogen can be produced both on a small scale, that is, on local level, and massively - in large, centralized enterprises. The growth in hydrogen production will serve as an additional incentive to increase the supply of this substance for domestic purposes (for example, for heating houses and offices).
Subscribe to our feeds
Similar to the existence of different types of internal combustion engines, there are different types of fuel cells - the choice of the appropriate type of fuel cell depends on its application.
Fuel cells are divided into high temperature and low temperature. Low Temperature Fuel Cells require relatively pure hydrogen as fuel. This often means that fuel processing is required to convert the primary fuel (such as natural gas) to pure hydrogen. This process consumes additional energy and requires special equipment. High Temperature Fuel Cells do not need this additional procedure, as they can "internally convert" the fuel at elevated temperatures, which means that there is no need to invest in hydrogen infrastructure.
Fuel cells on molten carbonate (MCFC)

Molten carbonate electrolyte fuel cells are high temperature fuel cells. The high operating temperature allows direct use of natural gas without a fuel processor and fuel gas low calorific value fuels from industrial processes and other sources. This process was developed in the mid 1960s. Since that time, manufacturing technology, performance and reliability have been improved.
The operation of RCFC is different from other fuel cells. These cells use an electrolyte from a mixture of molten carbonate salts. Currently, two types of mixtures are used: lithium carbonate and potassium carbonate or lithium carbonate and sodium carbonate. To melt carbonate salts and achieve a high degree of mobility of ions in the electrolyte, the operation of fuel cells with molten carbonate electrolyte occurs at high temperatures(650°C). The efficiency varies between 60-80%.
When heated to a temperature of 650°C, salts become a conductor for carbonate ions (CO 3 2-). These ions pass from the cathode to the anode where they combine with hydrogen to form water, carbon dioxide and free electrons. These electrons are sent through an external electrical circuit back to the cathode, generating electrical current and heat as a by-product.
Anode reaction: CO 3 2- + H 2 => H 2 O + CO 2 + 2e -
Reaction at the cathode: CO 2 + 1 / 2 O 2 + 2e - => CO 3 2-
General element reaction: H 2 (g) + 1/2 O 2 (g) + CO 2 (cathode) => H 2 O (g) + CO 2 (anode)
The high operating temperatures of molten carbonate electrolyte fuel cells have certain advantages. At high temperatures, the natural gas is internally reformed, eliminating the need for a fuel processor. In addition, the advantages include the ability to use standard materials of construction, such as stainless steel sheet and nickel catalyst on the electrodes. The waste heat can be used to generate high pressure steam for various industrial and commercial applications.
High reaction temperatures in the electrolyte also have their advantages. The use of high temperatures takes a long time to reach optimal operating conditions, and the system reacts more slowly to changes in energy consumption. These characteristics allow the use of fuel cell systems with molten carbonate electrolyte in constant power conditions. High temperatures prevent fuel cell damage by carbon monoxide, "poisoning", etc.
Molten carbonate fuel cells are suitable for use in large stationary installations. Industrially produced thermal power plants with output electric power 2.8 MW. Plants with an output power of up to 100 MW are being developed.
Phosphoric Acid Fuel Cells (PFC)

Fuel cells based on phosphoric (orthophosphoric) acid were the first fuel cells for commercial use. This process was developed in the mid-1960s and has been tested since the 1970s. Since then, stability, performance and cost have been increased.
Fuel cells based on phosphoric (orthophosphoric) acid use an electrolyte based on orthophosphoric acid (H 3 PO 4) with a concentration of up to 100%. The ionic conductivity of phosphoric acid is low at low temperatures, for this reason these fuel cells are used at temperatures up to 150–220°C.
Charge carrier in fuel cells of this type is hydrogen (H + , proton). A similar process occurs in proton exchange membrane fuel cells (MEFCs), in which hydrogen supplied to the anode is split into protons and electrons. The protons pass through the electrolyte and combine with oxygen from the air at the cathode to form water. The electrons are directed along an external electrical circuit, and an electric current is generated. Below are the reactions that generate electricity and heat.
Reaction at the anode: 2H 2 => 4H + + 4e -
Reaction at the cathode: O 2 (g) + 4H + + 4e - \u003d\u003e 2H 2 O
General element reaction: 2H 2 + O 2 => 2H 2 O
The efficiency of fuel cells based on phosphoric (orthophosphoric) acid is more than 40% when generating electrical energy. In the combined production of heat and electricity, the overall efficiency is about 85%. In addition, given operating temperatures, waste heat can be used to heat water and generate steam at atmospheric pressure.
The high performance of thermal power plants on fuel cells based on phosphoric (orthophosphoric) acid in the combined production of heat and electricity is one of the advantages of this type of fuel cells. The plants use carbon monoxide at a concentration of about 1.5%, which greatly expands the choice of fuel. In addition, CO 2 does not affect the electrolyte and the operation of the fuel cell, this type of cell works with reformed natural fuel. Simple design, low electrolyte volatility and increased stability are also advantages of this type of fuel cell.
Thermal power plants with an output electric power of up to 400 kW are industrially produced. Installations for 11 MW have passed the relevant tests. Plants with an output power of up to 100 MW are being developed.
Fuel Cells with Proton Exchange Membrane (PME)

Proton exchange membrane fuel cells are considered the best type of fuel cells for vehicle power generation, which can replace gasoline and diesel internal combustion engines. These fuel cells were first used by NASA for the Gemini program. Today, installations on MOPFC with a power of 1 W to 2 kW are being developed and demonstrated.
These fuel cells use a solid polymer membrane (thin plastic film) as the electrolyte. When impregnated with water, this polymer passes protons, but does not conduct electrons.
The fuel is hydrogen, and the charge carrier is a hydrogen ion (proton). At the anode, the hydrogen molecule is separated into a hydrogen ion (proton) and electrons. The hydrogen ions pass through the electrolyte to the cathode, while the electrons move around the outer circle and produce electrical energy. Oxygen, which is taken from the air, is fed to the cathode and combines with electrons and hydrogen ions to form water. The following reactions take place on the electrodes:
Reaction at the anode: 2H 2 + 4OH - => 4H 2 O + 4e -
Reaction at the cathode: O 2 + 2H 2 O + 4e - \u003d\u003e 4OH -
General element reaction: 2H 2 + O 2 => 2H 2 O
Compared to other types of fuel cells, proton exchange membrane fuel cells produce more power for a given fuel cell volume or weight. This feature allows them to be compact and lightweight. In addition, the operating temperature is less than 100°C, which allows you to quickly start operation. These characteristics, as well as the ability to quickly change energy output, are just some of the features that make these fuel cells a prime candidate for use in vehicles.
Another advantage is that the electrolyte is a solid rather than a liquid substance. Keeping the gases at the cathode and anode is easier with a solid electrolyte and therefore such fuel cells are cheaper to produce. Compared to other electrolytes, the use of a solid electrolyte does not cause problems such as orientation, there are fewer problems due to the occurrence of corrosion, which leads to a longer durability of the cell and its components.
Solid oxide fuel cells (SOFC)

Solid oxide fuel cells are the fuel cells with the highest operating temperature. The operating temperature can vary from 600°C to 1000°C, which allows the use of various types of fuel without special pre-treatment. To handle these high temperatures, the electrolyte used is a thin ceramic-based solid metal oxide, often an alloy of yttrium and zirconium, which is a conductor of oxygen (O 2 -) ions. The technology of using solid oxide fuel cells has been developing since the late 1950s. and has two configurations: planar and tubular.
A solid electrolyte provides a hermetic gas transition from one electrode to another, while liquid electrolytes are located in a porous substrate. The charge carrier in fuel cells of this type is the oxygen ion (O 2 -). At the cathode, oxygen molecules are separated from the air into an oxygen ion and four electrons. Oxygen ions pass through the electrolyte and combine with hydrogen to form four free electrons. Electrons are directed through an external electrical circuit, generating electrical current and waste heat.
Reaction at the anode: 2H 2 + 2O 2 - => 2H 2 O + 4e -
Reaction at the cathode: O 2 + 4e - => 2O 2 -
General element reaction: 2H 2 + O 2 => 2H 2 O
The efficiency of the generated electrical energy is the highest of all fuel cells - about 60%. In addition, high operating temperatures allow for combined heat and power generation to generate high pressure steam. Combining a high-temperature fuel cell with a turbine creates a hybrid fuel cell to increase the efficiency of electrical power generation by up to 70%.
Solid oxide fuel cells operate at very high temperatures (600°C-1000°C), resulting in a long time to reach optimal operating conditions, and the system is slower to respond to changes in power consumption. At such high operating temperatures, no converter is required to recover hydrogen from the fuel, allowing the thermal power plant to operate with relatively impure fuels from coal gasification or waste gases, and the like. Also, this fuel cell is excellent for working with high power, including industrial and large central power plants. Industrially produced modules with an output electrical power of 100 kW.
Fuel cells with direct methanol oxidation (DOMTE)
The technology of using fuel cells with direct oxidation of methanol is undergoing a period of active development. It has successfully established itself in the field of powering mobile phones, laptops, as well as for creating portable power sources. what the future application of these elements is aimed at.
The structure of fuel cells with direct oxidation of methanol is similar to fuel cells with a proton exchange membrane (MOFEC), i.e. a polymer is used as an electrolyte, and a hydrogen ion (proton) is used as a charge carrier. However, liquid methanol (CH 3 OH) is oxidized in the presence of water at the anode, releasing CO 2 , hydrogen ions and electrons, which are guided through an external electrical circuit, and an electric current is generated. Hydrogen ions pass through the electrolyte and react with oxygen from the air and electrons from the external circuit to form water at the anode.
Reaction at the anode: CH 3 OH + H 2 O => CO 2 + 6H + + 6e -
Reaction at the cathode: 3 / 2 O 2 + 6H + + 6e - => 3H 2 O
General element reaction: CH 3 OH + 3/2 O 2 => CO 2 + 2H 2 O
The development of these fuel cells began in the early 1990s. After the development of improved catalysts, and thanks to other recent innovations, power density and efficiency have been increased up to 40%.
These elements were tested in the temperature range of 50-120°C. With low operating temperatures and no need for a converter, direct methanol fuel cells are the best candidate for both mobile phones and other consumer goods, as well as in car engines. The advantage of this type of fuel cells is their small size, due to the use of liquid fuel, and the absence of the need to use a converter.
Alkaline fuel cells (AFC)

Alkaline fuel cells (ALFCs) are one of the most studied technologies and have been used since the mid-1960s. by NASA in the Apollo and Space Shuttle programs. On board these spacecraft, fuel cells produce electricity and drinking water. Alkaline fuel cells are one of the most efficient elements used to generate electricity, with power generation efficiency reaching up to 70%.
Alkaline fuel cells use an electrolyte, i.e. an aqueous solution of potassium hydroxide, contained in a porous, stabilized matrix. The concentration of potassium hydroxide may vary depending on the operating temperature of the fuel cell, which ranges from 65°C to 220°C. The charge carrier in an SFC is a hydroxide ion (OH-) moving from the cathode to the anode where it reacts with hydrogen to produce water and electrons. The water produced at the anode moves back to the cathode, again generating hydroxide ions there. As a result of this series of reactions taking place in the fuel cell, electricity is produced and, as a by-product, heat:
Reaction at the anode: 2H 2 + 4OH - => 4H 2 O + 4e -
Reaction at the cathode: O 2 + 2H 2 O + 4e - \u003d\u003e 4OH -
General reaction of the system: 2H 2 + O 2 => 2H 2 O
The advantage of SFCs is that these fuel cells are the cheapest to produce, since the catalyst needed on the electrodes can be any of the substances that are cheaper than those used as catalysts for other fuel cells. In addition, SCFCs operate at relatively low temperatures and are among the most efficient fuel cells - such characteristics can accordingly accelerate power generation and high efficiency fuel.
One of the characteristic features of SHTE is its high sensitivity to CO 2 , which can be contained in fuel or air. CO 2 reacts with the electrolyte, quickly poisons it, and greatly reduces the efficiency of the fuel cell. Therefore, the use of SFCs is limited to closed spaces such as space and underwater vehicles, they must operate on pure hydrogen and oxygen. Moreover, molecules such as CO, H 2 O and CH 4 , which are safe for other fuel cells and even fuel for some of them, are detrimental to SFC.
Polymer electrolyte fuel cells (PETE)

In the case of polymer electrolyte fuel cells, the polymer membrane consists of polymer fibers with water regions in which there is a conduction of water ions H 2 O + (proton, red) attached to the water molecule. Water molecules present a problem due to slow ion exchange. Therefore, a high concentration of water is required both in the fuel and on the exhaust electrodes, which limits the operating temperature to 100°C.
Solid acid fuel cells (SCFC)

In solid acid fuel cells, the electrolyte (C s HSO 4 ) does not contain water. The operating temperature is therefore 100-300°C. The rotation of the SO 4 2- oxy anions allows the protons (red) to move as shown in the figure. Typically, a solid acid fuel cell is a sandwich in which a very thin layer of solid acid compound is sandwiched between two tightly compressed electrodes to ensure good contact. When heated, the organic component evaporates, leaving through the pores in the electrodes, retaining the ability of numerous contacts between the fuel (or oxygen at the other end of the cell), electrolyte and electrodes.

| Fuel cell type | Working temperature | Power Generation Efficiency | Fuel type | Application area |
|---|---|---|---|---|
| RKTE | 550–700°C | 50-70% | Medium and large installations | |
| FKTE | 100–220°C | 35-40% | pure hydrogen | Large installations |
| MOPTE | 30-100°C | 35-50% | pure hydrogen | Small installations |
| SOFC | 450–1000°C | 45-70% | Most hydrocarbon fuels | Small, medium and large installations |
| POMTE | 20-90°C | 20-30% | methanol | Portable units |
| SHTE | 50–200°C | 40-65% | pure hydrogen | space research |
| PETE | 30-100°C | 35-50% | pure hydrogen | Small installations |