Encyclopedic YouTube
-
1 / 5
Mathematically, the definition of efficiency can be written as:
η = A Q , (\displaystyle \eta =(\frac (A)(Q)),)Where A- useful work (energy), and Q- wasted energy.
If the efficiency is expressed as a percentage, then it is calculated by the formula:
η = A Q × 100 % (\displaystyle \eta =(\frac (A)(Q))\times 100\%) ε X = Q X / A (\displaystyle \varepsilon _(\mathrm (X) )=Q_(\mathrm (X) )/A),Where Q X (\displaystyle Q_(\mathrm (X) ))- heat taken from the cold end (refrigeration capacity in refrigeration machines); A (\displaystyle A)
For heat pumps use the term transformation ratio
ε Γ = Q Γ / A (\displaystyle \varepsilon _(\Gamma )=Q_(\Gamma )/A),Where Q Γ (\displaystyle Q_(\Gamma ))- condensation heat transferred to the coolant; A (\displaystyle A)- the work (or electricity) spent on this process.
In the perfect car Q Γ = Q X + A (\displaystyle Q_(\Gamma )=Q_(\mathrm (X) )+A), hence for perfect car ε Γ = ε X + 1 (\displaystyle \varepsilon _(\Gamma )=\varepsilon _(\mathrm (X) )+1)
best performance performance for refrigeration machines has a reverse Carnot cycle: in it the coefficient of performance
ε = T X T Γ − T X (\displaystyle \varepsilon =(T_(\mathrm (X) ) \over (T_(\Gamma )-T_(\mathrm (X) )))), since, in addition to the energy taken into account A(e.g. electrical), to heat Q there is also energy taken from a cold source.The work done by the engine is:
This process was first considered by the French engineer and scientist N. L. S. Carnot in 1824 in the book Reflections on the driving force of fire and on machines capable of developing this force.
The purpose of Carnot's research was to find out the reasons for the imperfection of heat engines of that time (they had an efficiency of ≤ 5%) and to find ways to improve them.
The Carnot cycle is the most efficient of all. Its efficiency is maximum.

The figure shows the thermodynamic processes of the cycle. In the process of isothermal expansion (1-2) at a temperature T 1 , the work is done by changing the internal energy of the heater, i.e., by supplying the amount of heat to the gas Q:
A 12 = Q 1 ,
Cooling of the gas before compression (3-4) occurs during adiabatic expansion (2-3). Change in internal energy ΔU 23 in an adiabatic process ( Q=0) is completely converted into mechanical work:
A 23 = -ΔU 23 ,
The temperature of the gas as a result of adiabatic expansion (2-3) decreases to the temperature of the refrigerator T 2 < T 1 . In the process (3-4), the gas is isothermally compressed, transferring the amount of heat to the refrigerator Q2:
A 34 = Q 2,
The cycle is completed by the process of adiabatic compression (4-1), in which the gas is heated to a temperature T 1.
The maximum value of the efficiency of heat engines operating on ideal gas, according to the Carnot cycle:
 .
.The essence of the formula is expressed in the proven WITH. Carnot theorem that the efficiency of any heat engine cannot exceed cycle efficiency Carnot carried out at the same temperature of the heater and refrigerator.
Efficiency (efficiency) - a characteristic of the efficiency of a system (device, machine) in relation to the conversion or transfer of energy. It is determined by the ratio of useful energy used to the total amount of energy received by the system; usually denoted η ("this"). η = Wpol/Wcym. Efficiency is a dimensionless quantity and is often measured as a percentage. Mathematically, the definition of efficiency can be written as:
X 100%
Where A- useful work, and Q- wasted energy.
By virtue of the law of conservation of energy, the efficiency is always less than unity or equal to it, that is, it is impossible to obtain more useful work than the energy expended.
Heat engine efficiency- relation of the perfect useful work engine, to the energy received from the heater. The efficiency of a heat engine can be calculated using the following formula
,where - the amount of heat received from the heater, - the amount of heat given to the refrigerator. The highest efficiency among cyclic machines operating at given hot spring temperatures T 1 and cold T 2 , have heat engines, working on the Carnot cycle ; this limiting efficiency is equal to
.Not all indicators characterizing the efficiency of energy processes correspond to the above description. Even if they are traditionally or erroneously called "", they may have other properties, in particular, exceed 100%.
boiler efficiency
Main article: Boiler thermal balance
The efficiency of fossil fuel boilers is traditionally calculated from the net calorific value; it is assumed that the moisture of the combustion products leaves the boiler in the form of superheated steam. In condensing boilers, this moisture is condensed, the heat of condensation is usefully used. At efficiency calculation in terms of lower calorific value, it can eventually turn out to be more than one. IN this case it would be more correct to consider it according to the higher calorific value, taking into account the heat of steam condensation; however, the performance of such a boiler is difficult to compare with data from other installations.
Heat pumps and chillers
The advantage of heat pumps as a heating technique is the ability to sometimes receive more heat than the energy spent on their work; similarly, a refrigeration machine can remove more heat from the cooled end than is expended in organizing the process.
The efficiency of such heat engines is characterized by coefficient of performance(for chillers) or transformation ratio(for heat pumps)
,where is the heat taken from the cold end (in refrigeration machines) or transferred to the hot end (in heat pumps); - the work (or electricity) spent on this process. The best performance indicators for such machines have the reverse Carnot cycle: in it the coefficient of performance
,where , are the temperatures of the hot and cold ends, . This value, obviously, can be arbitrarily large; although practically it is difficult to approach it, the coefficient of performance can still exceed unity. This does not contradict the first law of thermodynamics, since, in addition to the energy taken into account A(e.g. electric), into heat Q there is also energy taken from a cold source.
Literature
- Peryshkin A.V. Physics. 8th grade. - Bustard, 2005. - 191 p. - 50,000 copies. - ISBN 5-7107-9459-7.
Notes
Wikimedia Foundation. 2010 .
Synonyms:- TurboPascal
- efficiency
See what "" is in other dictionaries:
efficiency- The ratio of output power to consumed active power. [OST 45.55 99] coefficient useful action Efficiency A value that characterizes the perfection of the processes of transformation, transformation or transfer of energy, which is the ratio of useful ... ... Technical Translator's Handbook
EFFICIENCY- or coefficient of return (Efficiency) - a characteristic of the quality of the work of any machine or apparatus from the side of its efficiency. By K.P.D. is meant the ratio of the amount of work received from the machine or energy from the device to that amount ... ... Marine Dictionary
EFFICIENCY- (efficiency), an indicator of the efficiency of the mechanism, defined as the ratio of the work performed by the mechanism to the work expended on its functioning. efficiency usually expressed as a percentage. An ideal mechanism would have to have efficiency = ... ... Scientific and technical encyclopedic dictionary
EFFICIENCY Modern Encyclopedia
EFFICIENCY- (efficiency) characteristic of the efficiency of the system (device, machine) in relation to energy conversion; is determined by the ratio of useful energy used (turned into work in a cyclic process) to the total amount of energy, ... ... Big Encyclopedic Dictionary
EFFICIENCY- (efficiency), a characteristic of the efficiency of a system (device, machine) in relation to the conversion or transfer of energy; is determined by the ratio of t) useful energy used (Wpol) to the total amount of energy (Wtotal) received by the system; h=Wpol… … Physical Encyclopedia
EFFICIENCY- (efficiency) the ratio of useful energy W p, for example. in the form of work, to the total amount of energy W received by the system (machine or engine), W p / W. Due to the inevitable energy losses due to friction and other non-equilibrium processes for real systems ... ... Physical Encyclopedia
EFFICIENCY- the ratio of useful work expended or energy received to all work expended or energy consumed, respectively. For example, the efficiency of the electric motor is the ratio of mechan. the power they give off to the electric power supplied to it. power; TO.… … Technical railway dictionary
efficiency- noun, number of synonyms: 8 efficiency (4) return (27) fruitfulness (10) ... Synonym dictionary
Efficiency- - a value that characterizes the perfection of any system in relation to any process of transformation or energy transfer occurring in it, defined as the ratio of useful work to the work expended on putting into action. ... ... Encyclopedia of terms, definitions and explanations of building materials
Efficiency- (efficiency), a numerical characteristic of the energy efficiency of any device or machine (including a heat engine). Efficiency is determined by the ratio of useful energy used (i.e., converted into work) to the total amount of energy, ... ... Illustrated Encyclopedic Dictionary
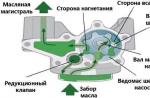
Probably everyone wondered about the efficiency (Coefficient of Efficiency) of the engine internal combustion. After all, the higher this indicator, the more efficient it works. power unit. The most efficient for this moment time is considered an electric type, its efficiency can reach up to 90 - 95%, but for internal combustion engines, whether it be diesel or gasoline, to put it mildly, it is far from ideal ...
To be honest, then modern options motors are much more efficient than their counterparts, which were released 10 years ago, and there are a lot of reasons for this. Think for yourself before the 1.6-liter option, it gave out only 60 - 70 hp. And now this value can reach 130 - 150 hp. This is painstaking work to increase efficiency, in which each "step" is given by trial and error. However, let's start with a definition.
is the value of the ratio of two quantities, the power that is supplied to crankshaft engine to the power received by the piston, due to the pressure of the gases that were formed by igniting the fuel.
In simple terms, this is the conversion of thermal or thermal energy that appears during combustion fuel mixture(air and gasoline) to mechanical. It should be noted that this has already happened, for example, with steam power plants- Also, the fuel under the influence of temperature pushed the pistons of the units. However, the installations there were many times larger, and the fuel itself was solid (usually coal or firewood), which made it difficult to transport and operate it, it was constantly necessary to “feed” it into the furnace with shovels. Internal combustion engines are much more compact and lighter than steam engines, and fuel is much easier to store and transport.
More about losses
Looking ahead, we can confidently say that the efficiency of a gasoline engine is in the range of 20 to 25%. And there are many reasons for this. If we take the incoming fuel and recalculate it as a percentage, then we kind of get “100% of the energy” that is transferred to the engine, and then the losses went:

1)Fuel efficiency . Not all fuel burns out, a small part of it leaves with exhaust gases, at this level we already lose up to 25% of efficiency. Of course now fuel systems improved, an injector appeared, but it is far from ideal.
2) The second is heat losses.And . The engine warms up itself and many other elements, such as radiators, its body, the liquid that circulates in it. Also, part of the heat is lost from exhaust gases. For all this, up to 35% loss of efficiency.
3) The third is mechanical losses . ON all kinds of pistons, connecting rods, rings - all places where there is friction. This includes losses from the load of the generator, for example, the more electricity the generator produces, the more it slows down the rotation of the crankshaft. Of course, lubricants have also stepped forward, but again, no one has yet completely defeated friction - another 20% loss
Thus, in the dry residue, the efficiency is about 20%! Of course, there are stand-out options from gasoline options, in which this figure is increased to 25%, but there are not so many of them.

That is, if your car consumes 10 liters of fuel per 100 km, then only 2 liters of them will go directly to work, and the rest are losses!
Of course, you can increase the power, for example, by boring the head, we are watching a short video.
If you remember the formula, you get:

Which engine has the highest efficiency?
Now I want to talk about gasoline and diesel options, and find out which one is the most efficient.
To put it simply, the language and not to climb into the wilds technical terms then - if we compare two efficiencies - the most efficient of them, of course, is diesel, and here's why:
1) A gasoline engine converts only 25% of energy into mechanical energy, but a diesel engine converts about 40%.
2) If equipped diesel type turbocharging, it is possible to achieve an efficiency of 50-53%, and this is very significant.

So why is it so effective? It's simple - despite the similar type of work (both are internal combustion units), a diesel engine does its job much more efficiently. It has greater compression, and the fuel ignites from a different principle. It heats up less, which means it saves on cooling, it has fewer valves (savings on friction), and it also doesn’t have the usual ignition coils and spark plugs, which means it doesn’t require additional energy costs from the generator. It works at lower speeds, you don’t need to spin the crankshaft furiously - it does all diesel variant efficiency champion.
About Diesel Fuel Efficiency
FROM more high value efficiency - followed by fuel efficiency. So, for example, a 1.6-liter engine can consume only 3-5 liters in the city, unlike petrol type, where the consumption is 7 - 12 liters. A diesel engine has a lot, the engine itself is often more compact and lighter, and also more environmentally friendly lately. All these positive moments are achieved due to the greater value, there is a direct relationship between efficiency and compression, see a small plate.

However, despite all the advantages, it also has many disadvantages.
As it becomes clear Engine efficiency internal combustion is far from ideal, so the future is clearly with electric options - it remains only to find efficient batteries that are not afraid of frost and hold a charge for a long time.
Efficiency, by definition, is the ratio of energy received to energy expended. If the engine burns gasoline and only a third of the generated heat is converted into energy for the movement of the car, then the efficiency is one-third, or (rounded up to whole) 33%. If a light bulb produces light energy fifty times less than the electrical energy consumed, its efficiency is 1/50 or 2%. However, here the question immediately arises: what if the light bulb is sold as an infrared heater? After the sale of incandescent lamps was banned, exactly the same design devices began to be sold as "infrared heaters", since over 95% of electricity is converted into heat.
(Imp) useful heat
Usually, the heat released during the operation of something is recorded as a loss. But this is far from certain. A power plant, for example, converts about a third of the heat released during the combustion of gas or coal into electricity, but another part of the energy can be used to heat water. If hot water supply and warm batteries are also included in the useful results of the CHP, then the efficiency will increase by 10-15%.
A similar example is an automobile "stove": it transfers part of the heat generated during engine operation to the passenger compartment. This heat can be useful and necessary, or it can be considered as a waste: for this reason, it usually does not appear in the efficiency calculations of an automobile motor.
Devices such as heat pumps stand apart. Their efficiency, if we consider it in terms of the ratio of heat produced and electricity consumed, is more than 100%, but this does not refute the foundations of thermodynamics. A heat pump pumps heat from a less heated body to a hotter one and expends energy on this, since without energy expenditure such a redistribution of heat is prohibited by the same thermodynamics. If a heat pump draws a kilowatt from an outlet and produces five kilowatts of heat, then four kilowatts will be drawn from the air, water, or soil outside the home. The environment in the place where the device draws heat from will cool down, and the house will warm up. But then this heat, together with the energy spent by the pump, will still dissipate in space.
The external circuit of the heat pump: liquid is pumped through these plastic pipes, taking heat from the water column into the heated building. Mark Johnson/Wikimedia
Much or effective?
Some devices are very high efficiency, but at the same time - inappropriate power.
Electric motors are more efficient the larger they are, but it is physically impossible and economically pointless to put an electric locomotive engine in a children's toy. Therefore, the efficiency of engines in a locomotive exceeds 95%, and in a small radio-controlled car - at most 80%. And in the case of electric motor its efficiency also depends on the load: an underloaded or overloaded motor works with less efficiency. Correct selection equipment can mean even more than just choosing a device with the maximum declared efficiency.

The most powerful serial locomotive, Swedish IORE. The second place is held by the Soviet electric locomotive VL-85. Kabelleger/Wikimedia
If electric motors are produced for a variety of purposes, from vibrators in phones to electric locomotives, then the ion engine has a much smaller niche. Ion thrusters efficient, economical, durable (work without shutdown for years), but turn on only in a vacuum and give very little thrust. They are ideal for sending scientific vehicles into deep space, which can fly to a target for several years and for which fuel savings are more important than time costs.
Electric motors, by the way, consume almost half of all the electricity generated by mankind, so even a difference of one hundredth of a percent on a global scale may mean the need to build another nuclear reactor or another power plant.
Effective or cheap?
Energy efficiency is not always identical to economic efficiency. illustrative example - LED bulbs, which until recently lost to incandescent and fluorescent "energy-saving" lamps. The complexity of manufacturing white LEDs, the high cost of raw materials and, on the other hand, the simplicity of an incandescent lamp forced us to choose less efficient, but cheap light sources.
By the way, for the invention of the blue LED, without which it would be impossible to make a bright white lamp, Japanese researchers received the Nobel Prize in 2014. This is not the first prize awarded for his contribution to the development of lighting: in 1912, Niels Dahlen, the inventor who improved the acetylene torches for lighthouses, was awarded.

Blue LEDs are needed to produce white light in combination with red and green. These two colors have learned to get enough bright LEDs much earlier; blue for a long time remained too dull and expensive for mass use
Another example of effective but very expensive devices- solar cells based on gallium arsenide (semiconductor with formula GaAs). Their efficiency reaches almost 30%, which is one and a half to two times higher than batteries used on Earth based on much more common silicon. High efficiency justifies itself only in space, where the delivery of one kilogram of cargo can cost almost as much as a kilogram of gold. Then saving on the mass of the battery will be justified.
The efficiency of power lines can be improved by replacing copper with silver, which is better conductive, but silver cables are too expensive and therefore used only in isolated cases. But to the idea of building superconducting power lines from expensive rare-earth ceramics that require cooling with liquid nitrogen in last years applied several times in practice. In particular, such a cable has already been laid and connected in the German city of Essen. It is rated at 40 megawatts electrical power at ten kilovolts. In addition to the fact that heating losses are reduced to zero (however, cryogenic installations need to be powered instead), such a cable is much more compact than usual and due to this you can save on buying expensive land in the city center or refuse to lay additional tunnels.
Not according to general rules
From the school course, many remember that the efficiency cannot exceed 100% and that it is the higher, the more difference temperature between refrigerator and heater. However, this is true only for so-called heat engines: Steam engine, internal combustion engine, jet and rocket engines, gas and steam turbines.
Electric motors and all electrical devices this rule is not obeyed because they do not thermal machines. For them, it is only true that the efficiency cannot exceed one hundred percent, and particular restrictions are defined differently in each case.
In the case of a solar battery, the losses are determined both by quantum effects during the absorption of photons and by losses due to the reflection of light from the surface of the battery and to absorption in focusing mirrors. The calculations performed showed that to go beyond 90% solar battery cannot in principle, but in practice values of about 60-70% are achievable, and even those with a very complex structure of photocells.
Fuel cells have excellent efficiency. These devices receive some substances that enter into a chemical reaction with each other and give an electric current. This process, again, is not a heat engine cycle, so the efficiency is quite high, about 60%, while diesel or Gas engine do not usually go beyond 50%.
It was the fuel cells that were on those flying to the moon spaceships"Apollo", and they can work, for example, on hydrogen and oxygen. Their only drawback is that hydrogen must be sufficiently pure and, moreover, it must be stored somewhere and somehow transferred from the plant to consumers. Technologies that allow hydrogen to replace ordinary methane have not yet been brought to mass use. on hydrogen and fuel cells only experimental vehicles and a number of submarines are in operation.

Plasma engines of the SPD series. They are made by OKB Fakel, and they are used to keep satellites in a given orbit. Thrust is created by the flow of ions that occur after the ionization of an inert gas electrical discharge. The efficiency of these engines reaches 60 percent
Ion and plasma engines already exist, but they also only work in a vacuum. In addition, their thrust is too small and orders of magnitude lower than the weight of the device itself - they would not take off from the Earth even in the absence of an atmosphere. But during interplanetary flights lasting many months and even years, weak thrust is compensated by efficiency and reliability.